32+ energy of activation calculator
Use Trial 2 in Part A for the concentrations of the reactants. Find out the activation energy considering that k 275 X10-2Lmol Also.

It Takes A Dimer To Tango Oligomeric Small Heat Shock Proteins Dissociate To Capture Substrate Biorxiv
E a the activation energy of the reaction in Jmol.
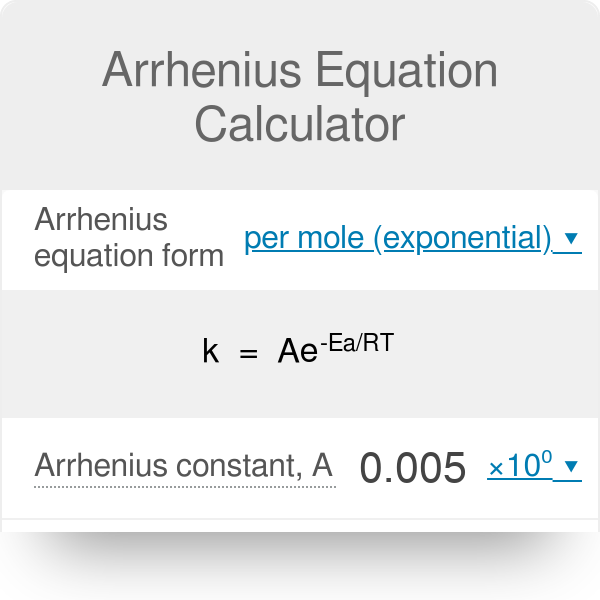
. Web This calculator uses neutron cross sections to compute activation on the sample given the mass in the sample and the time in the beam or to perform scattering. Chemistry questions and answers. Web The dependence of the rate constant on activation energy and temperature can be expressed in the Arrhenius Equation.
Web The Arrhenius Activation Energy for Two Temperature calculator uses the Arrhenius equation to compute activation energy based on two temperatures and two reaction rate. Hence Ea 275819 kJmol. R the ideal gas constant 83145 JKmol.
Web To use this online calculator for Activation Energy for Backward Reaction enter Activation Energy Forward E af Enthalpy of Reaction ΔH and hit the calculate. 221 k A e E a R T. Where k represents the rate constant Ea is the.
32 Calculate the energy of activation with the following data. Web In order to calculate the activation energy we need an equation that relates the rate constant of a reaction with the temperature energy of the system. Calculation of Energy of Activation.
T283 K T2 303 K rate constant ki 93 x 10 and. One can also derive the activation energy formula in an algebraic manner. It is an important parameter in many fields and applications as it represents an estimate.
Web The activation energy this required is Ea 1103276840. Web Now one use it to calculate the Activation Energy by making use of the graphing ink versus 1T. Web This type of question provides you with the pre-exponential factor or frequency factor which means you can calculate the activation energy at a single.
For the room temperature use the rate of Trial 2 in Part. Web Formula to calculate activation energy. Web In 1889 a Swedish scientist named Svante Arrhenius proposed an equation that relates these concepts with the rate constant.
Web 1 hour agoPart B. T 1 and T 2 absolute temperatures. Ea activation energy.
Web The activation energy is the minimum energy required to initiate a reaction.

It Takes A Dimer To Tango Oligomeric Small Heat Shock Proteins Dissociate To Capture Substrate Abstract Europe Pmc

Poster Presentations 2021 International Journal Of Rheumatic Diseases Wiley Online Library

Doublet Ground State In A Vanadium Ii Complex Redox And Coordinative Noninnocence Of Tripodal Ligand Architecture Inorganic Chemistry

Arrhenius Equation

Calculate The Activation Energy For A Reaction Of Which Rate Constants Becomes 4 Times When Temperature Changes From 30 0c To 50 0c R 8 314 J Mol 1k 1
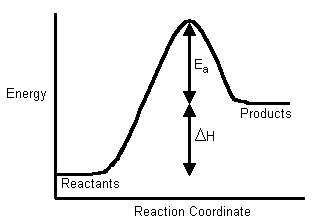
Activation Energy

Arrhenius Equation Calculator Online Solver With Free Steps

Calculating Activation Energy On Excel From Arrhenius Equation Youtube

Photon Energy Calculator Online Solver With Free Steps
The Arrhenius Equation
Copyright 2019 William M Holden

Effect Of Temperature And Pressure On The Kinetics Of The Oxygen Reduction Reaction The Journal Of Physical Chemistry A
Activation Energy
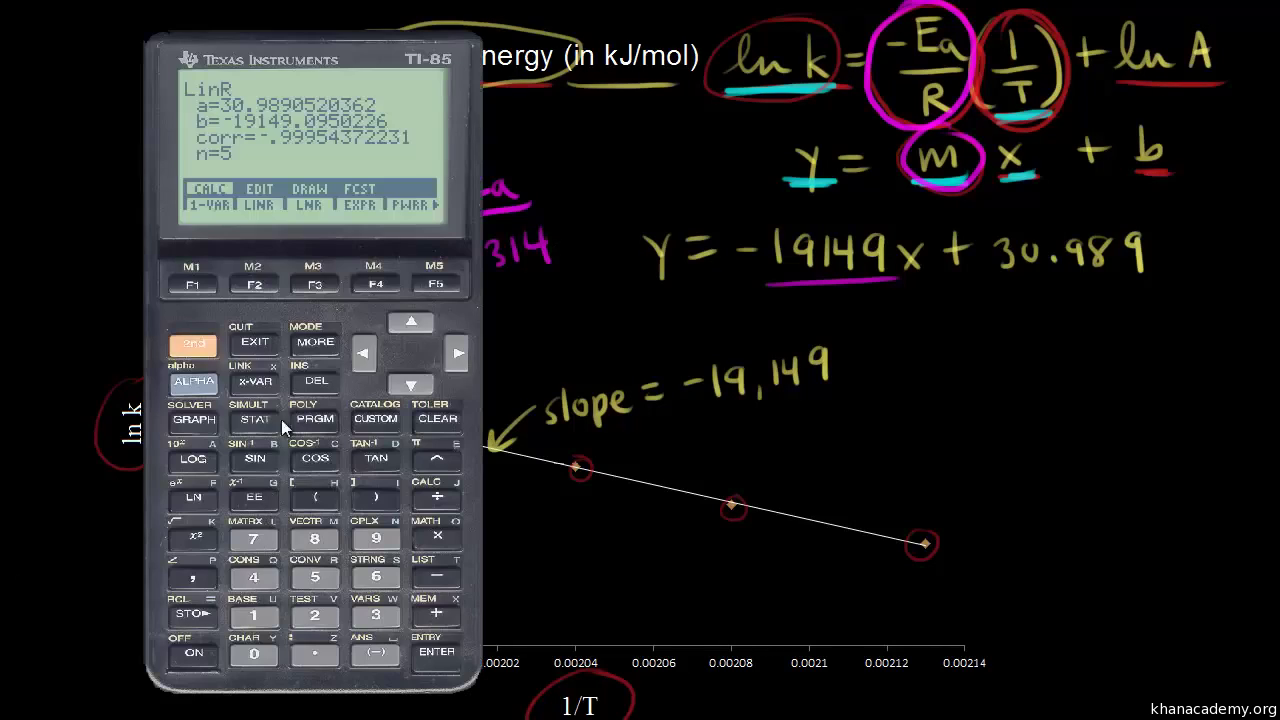
Using The Arrhenius Equation Video Khan Academy

Isokinetic Relationship Isoequilibrium Relationship And Enthalpy Entropy Compensation Chemical Reviews

Pdf On The Calculation Of Activation Energies And Frequency Factors From Glow Curves Semantic Scholar
Calculation Of Activation Energy From Ac Conductivity Ln R 1 T Scm A1 Download Scientific Diagram